Publié sur Medical Device Network le 3/03/2020.
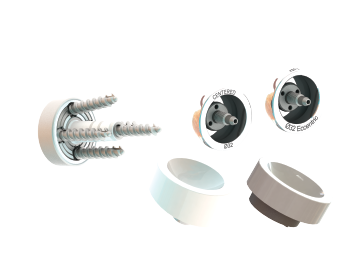
FX Shoulder has secured 510k clearance from the US Food and Drug Administration (FDA) for its Glenoid Baseplate with a central screw, 32mm glenosphere, and humeral cups.
The Glenoid Baseplate device is intended for reverse shoulder arthroplasty. It is claimed to be an alternative to the glenoid baseplate that the company currently provides to the US market.
[...]
FX Shoulder CEO Baptiste Martin said: These are significant achievements and additions for our portfolio. There is a constant need and request for exactly these types of implants.